Development
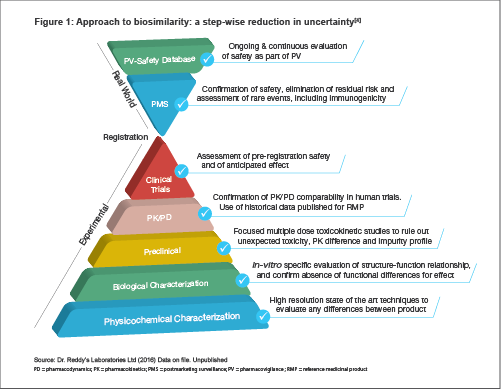
Our approach to biosimilarityThe World Health Organization (WHO) defines the essence of biosimilarity to be primarily 'the absence of a relevant difference in the parameter of interest'. The WHO Guidelines on Biosimilars, or Similar Bio-therapeutic Products, describes them as 'products which are similar in terms of quality, safety and efficacy to an already licensed reference biotherapeutic product.[1]
A biosimilar is thus comparable to its reference medicinal product, with respect to structural and functional equivalence and also clinical safety and efficacy.
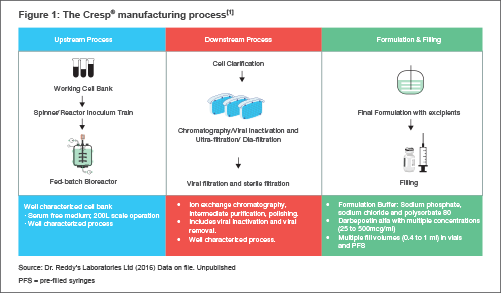
Dr. Reddy's standard approach to biosimilarity (as seen in Figure 1) conforms to the core development principles outlined by the WHO guidelines and regulatory agencies worldwide[2] i.e., tightly planned, comprehensive characterization and comparison at analytical and quality levels of both manufacturing process and product.[3] This sequentially eliminates any uncertainties about potential differences in efficacy, safety and patient outcome. Cresp development history
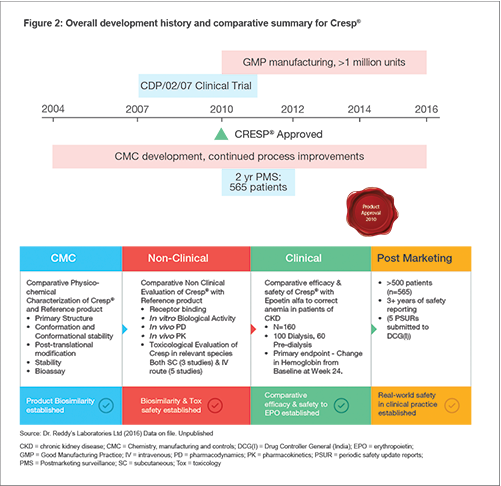
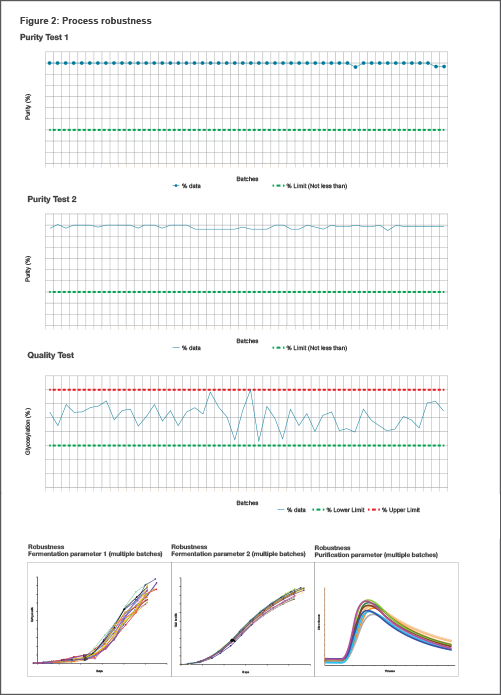
The development of Cresp® (see Figure 2), a biosimilar darbepoetin alfa, was based on gaining an understanding of the Reference Product based on information in the literature and testing of multiple reference lots to identify all relevant product quality attributes. These quality targets served as the basis for process development activities and defined the selection of process steps, material attributes and process parameter controls for the manufacturing process.[4] Structure-Functional-Clinical Equivalence of Cresp®
Cresp® : proof of biosimilarity
A thorough understanding of the critical quality attributes of the Reference Product, and a rigorous set of analytical testing assays are central to any comparison exercise with biosimilar products. Our understanding of the Reference Product is built on studying the scientific literature, and continuous characterization of several lots of the Reference Product, using an array of advanced analytical methods. These methods deliver a detailed picture of the physical, chemical and biological characteristics of both the Reference and biosimilar Products.[2]
Biopharmaceuticals, such as darbepoetin alfa, produced using biological systems, can exhibit batch-to-batch variations[5] in product attributes that are influenced by the process. These are controlled to be within a range by adequate process controls, that, from current knowledge, will entail no adverse impact on safety or efficacy. [6] The product attributes that display batch-to-batch variation can be concluded to be 'similar' whereas fixed attributes such as the protein sequence can be concluded as 'identical'. The conclusion of similarity is based on the data sets for Cresp® and the Reference Product being largely overlapping, based on assessment of multiple lots of each product.
Establishing structural equivalence
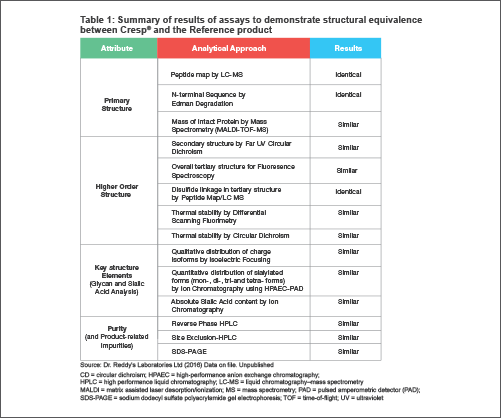
An extensive array of sophisticated analytical physicochemical methods have been used to compare protein structural elements at various orders in a hierarchy, such as product variants due to post-translational modifications and product purity profiles. The results are summarized in Table 1.[4]
A: Primary structure and overall mass - Indicates identical amino acid sequence, and is the key determinant of all other molecular properties, including functional activity and stability. This is verified first using nucleotide sequencing for ensuring coding for the correct amino acid sequence, and then demonstrated at the protein level by a peptide map covering the complete sequence. The key residue positions of carbohydrate attachments are also confirmed using a combination of chromatographic and mass spectrometric techniques (LC-MS).[4]
B: Intact mass determined by mass spectrometry demonstrates that the overall mass of Cresp® and the Reference Product is highly comparable. About 50% of the mass is contributed by carbohydrates, the mass and the structure of which are deeply influenced by manufacturing. Mass data with multiple batches of the products also show that the manufacturing process is robust in producing Cresp® consistently.[4]
C: Multiple lots of Cresp® and Reference Product subjected to circular dichroism (CD) analysis in the far ultraviolet (UV) region showed superimposable spectra indicating that the products have highly similar secondary structures.
D: Variations in protein intrinsic fluorescence in the 300–450 nm range, primarily contributed by tryptophan residues, are widely used as a diagnostic for perturbations in the local conformations and global tertiary structures. Data with Cresp® and Reference Product show superimposable fluorescence spectra, indicating similar tertiary structures.[4]
E: Intrinsic thermal stability of proteins determined by CD spectroscopy demonstrates that Cresp® is highly comparable to the Reference Product indicating a similarly folded and stable structure.
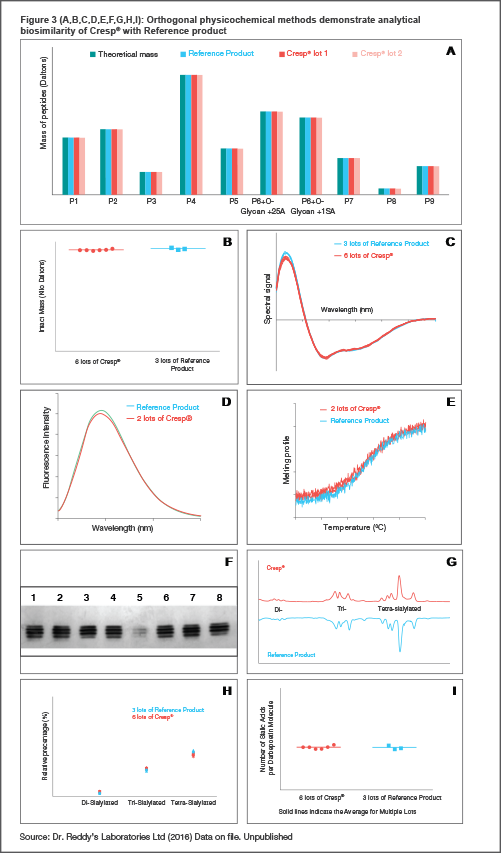
Sialic acid moieties in the carbohydrate content in darbepoetin alfa greatly influence its physical and biological properties. The number of sialic acid units per protein molecule, and the branching structure and nature of sialic acid residues, impact in vivo biological activity, clinical pharmacokinetics and pharmacodynamics.[7] Hence, demonstration of comparability with respect to sialic acids is key to establishing biosimilarity.
A complete assessment of the distribution of sialic acids and the average sialic acid content are key to the analysis of this attribute, and can be achieved only through the use of multiple, independent analytical methods: gel-based isoelectric focusing (IEF) and two ion chromatography-based methods using high-performance anion exchange chromatography (HPAEC) with a pulsed amperometric detector (PAD). We evaluated multiple lots of Cresp® and the Reference Product head-to-head in all these assays.
F: Gel based Isoelectric focussing (IEF) is used to assess the levels of sialylated isoforms of darbepoetin alfa. The characteristic band pattern observed is due to the charged carbohydrate structures (with terminal sialic acid) linked to the amino acids and is deeply influenced by the manufacturing process. The dominant product isoforms in Cresp® (lanes 1,3,6,8 in Figure 3F) and the Reference Product (lanes 2,7 in Figure 3F), each represented by a band, are observed to be same across multiple lots[4]
G, H and I: HPAEC with a PAD are necessary to evaluate the quantitative distribution of sialylated isoforms and to estimate the sialic acid content per protein molecule. Relative distribution of the mono-, di, tri and tetra-sialylated isoforms as well as the quantitative analysis of the amount of sialic acid show high comparability between Cresp® and the Reference Product.
Conclusions for structural similarity
Considering the results from multiple different assays (Figure 3, panels A-I) performed to evaluate primary, secondary, tertiary structural features and carbohydrate modifications, it can be concluded that Cresp® is highly similar to the Reference Product with respect to sialylation—a critical quality attributed with clinical significance. In addition, given that the carbohydrate modifications are significantly influenced by the manufacturing process, the consistency of multiple lots of Cresp® with regard to this critical quality attribute demonstrates the robustness of the Cresp® manufacturing process.
Establishing functional equivalence
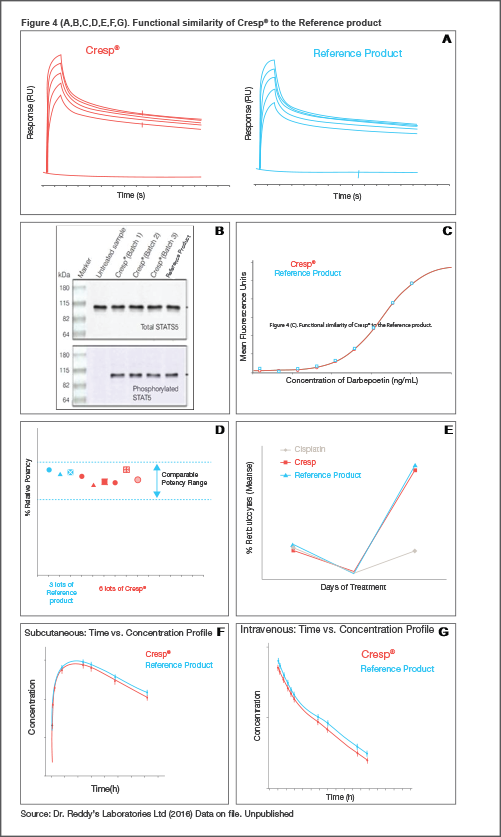
The functional equivalence of Cresp® with the Reference Product was established in terms of the mechanism of action at the molecular level (receptor binding, signalling inside the cell, biological activity), and their pharmacodynamic and pharmacokinetic profiles in animal models. Data showed that they were highly similar in action, potency, the ability to induce a physiological response and clearance inside the body. A: Receptor Binding - Darbepoetin alfa binds to the transmembrane erythropoietin receptor (EPO-R) on hematopoietic progenitor cells, the first step in the signal transduction cascade that leads to a physiological response. This binding event between EPO-R and Cresp® (or Reference Product) at different concentrations was assessed in real-time by using Surface Plasmon Resonance technology. Data from the association and the dissociation phases show that Cresp® and Reference Product bind to the target (EPO-R) in a very similar manner.[4]
B: Qualitative Western Blot (was used to determine the phosphorylation of STAT5, a key step in the signalling cascade upon binding to EPO receptor. Comparable levels of phopsporylated STAT5 were found in multiple lots of Cresp and Reference Product.
C: Quantitative cell based assays are used to assess the proliferation of cells in a dose dependent manner. The overlapping dose response curves of Cresp® and Reference Product indicate highly similar biological activity.
D: The potency values, derived from the cell based proliferation assay for multiple lots of Cresp® and the Reference Product are also highly comparable.[4]
E: Assessment of Pharmacodynamics: In vivo pharmacodynamics studies were conducted to compare increases in reticulocyte counts in healthy mice or anemic (cisplatin induced) Wistar rat models (E) in response to Cresp® or Reference Product. Highly comparable increase in reticulocyte counts were observed for both, confirming similarity of biological activity.
F & G: Pharmacokinetics analyses: We conducted pharmacokinetics studies to examine the absorption, distribution, metabolism and excretion of the drug were conducted by administering a single subcutaneous (F) or an intravenous dose (G) of Cresp® or the Reference Product to normal Wistar rats, indicated high comparability in pharmacokinetic profiles for Cresp® and Reference Product, with overlapping concentration curves and systemic exposure, half-life and clearance. Thus, Cresp® and the Reference Product have comparable in-vivo pharmacokinetics.
Summary of functional characterization
Based on results from target binding, signal transduction and cell proliferation assays, Cresp® and the Reference Product exhibit highly similar biological activity. [4] The ability to induce a physiological response from the body and clearance mechanism were also confirmed to be very similar for Cresp® and the Reference Product.[4]
Establishing clinical equivalence
In addition to the structural, functional, and preclinical evaluation, the clinical evaluation of Cresp® is an essential process in assessing its safety and efficacy in patients of anemia with chronic kidney disease (CKD). It was observed that Cresp® has a risk-benefit profile similar to that of darbepoetin alfa (Aranesp®) reported in the literature. There was a satisfactory rise and maintenance of hemoglobin levels both in pre-dialysis and dialysis patients of CKD.
Data was presented at the Indian Society of Nephrology Conference (ISNCON) 2014, at Kolkata, India:
- Dr. Gokulnath, Dr. KV Dakshinamurthy, Dr. Anupama Ramkumar, Dr. Luis Lopez Lazaro, Dr. Tazeen A Idris. A Randomized, Comparative, Open-label Clinical Trial of Darbepoetin Alfa Biosimilar for the Treatment of Anaemia in Pre-Dialysis Chronic Kidney Disease Patients (CDP/02/07). Poster #: 55, Abstract No. 0556, Indian Society of Nephrology Conference (ISNCON), 18 - 21 Dec 2014, Kolkata, India.
- Dr. Gokulnath, Dr. KV Dakshinamurthy, Dr. Anupama Ramkumar, Dr. Luis Lopez Lazaro, Dr. Tazeen A Idris. Efficacy and Safety of Indigenously Developed Darbepoetin Alfa in Dialysis Patients - A Randomised Study (CDP/02/07). Poster #: 16, Abstract No. 6010, Indian Society of Nephrology Conference (ISNCON), 18 - 21 Dec 2014, Kolkata, India.
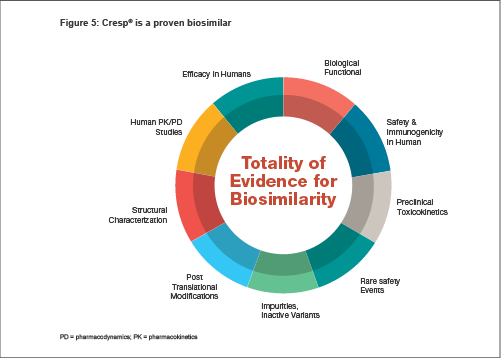
The evidence from the various physicochemical, functional, preclinical, clinical and real-life post-marketing studies (see Figure 5) performed shows that there are no clinically meaningful differences between Cresp® as a biological product and the Reference Product in terms of product safety, purity and potency.
CD = circular dichroism, CKD = chronic kidney disease, DNA = deoxyribonucleic acid, EPO = erythropoietin, EPO-R = erythropoietin receptor, HPAEC = high-performance anion exchange chromatography, IEF = isoelectric focusing, ISNCON = Indian Society of Nephrology Conference, LC-MS = liquid chromatography-mass spectrometry, PAD = pulsed amperometric detector, UV = Ultraviolet, WHO = World Health Organization
Cresp® is Dr. Reddy's brand of biosimilar darbepoetin alfa. Cresp® was registered in 2010 and until 2014, it was the only darbepoetin alfa licensed for use by physicians in India.
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product".
References
Cresp® is Dr. Reddy's brand of biosimilar darbepoetin alfa. Cresp® was registered in 2010 and until 2014, it was the only darbepoetin alfa licensed for use by physicians in India.
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product".
References
1. WHO Expert Committee on Biological Standardization. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). Sixtieth report (19-23 October 2009). WHO Technical Report Series No. 977, 2013 - Annex 2
2. Food and Drug Administration. Draft Guidance for Industry: Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. Food and Drug Administration. 2012.
3. Crommelin D et al. Eur J Hosp Pharm Sci. 2005;11(1):11-7.
4. Dr. Reddy's Laboratories Ltd (2016) Data on file. Unpublished
5. Schiestl M et al. Nat Biotechnol. 2011 Apr;29(4):310-2
6. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2004. Comparability of Biotechnological/ Biological products subject to changes in their manufacturing process Q5E.
7. Macdougall IC et al. Nephrol Dial Transplant. 2007 Jun;22 Suppl 4:iv2-iv9.
2. Food and Drug Administration. Draft Guidance for Industry: Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. Food and Drug Administration. 2012.
3. Crommelin D et al. Eur J Hosp Pharm Sci. 2005;11(1):11-7.
4. Dr. Reddy's Laboratories Ltd (2016) Data on file. Unpublished
5. Schiestl M et al. Nat Biotechnol. 2011 Apr;29(4):310-2
6. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2004. Comparability of Biotechnological/ Biological products subject to changes in their manufacturing process Q5E.
7. Macdougall IC et al. Nephrol Dial Transplant. 2007 Jun;22 Suppl 4:iv2-iv9.
Manufacturing, Quality & Supply Chain Capabilities
Manufacturing ProcessIn order to manufacture Cresp®, using genetic engineering, a gene coding for the darbepoetin alfa protein is introduced into Chinese Hamster Ovary cells (CHO) [identical to the Reference Product host cell line.[1]
The cells engineered to express this protein are grown under very specific and well-controlled conditions in a bioreactor. During the process, the cells multiply and synthesize the protein and release it into the surrounding growth medium. A series of purification and chromatographic steps then purify the protein from the medium to get highly purified darbepoetin alfa protein.
We formulate the purified protein with excipients that help to maintain product stability under recommended shelf life conditions. Manufacturing Robustness
Based on years of understanding of each unit operation, defined and validated process controls, and testing, we consistently ensure high product quality during manufacturing till the time the product reaches the patient.
Our strategy is to produce desired product quality consistently at scale, based on understanding each unit operation, specified and validated process controls, and process testing. Our robustness of process is proven (see Figure 2).[1] Quality
Our Cresp® manufacturing facility is approved by Drug Controller of General of India (DCGI) and few other global health authorities [1]. It is also certified by World Health Organization (WHO)-GMP and International Organization for Standardization (ISO) 9001.
Supply Chain Management Expertise Cold chain features
- Standard operating procedures for storage, packing, shipping and distribution based on good distribution practices.
- Partnership with globally renowned manufacturers of temperature controlled boxes and transport vendors.
- Audits and monitoring to check adherence to laid down procedures and to make continuous improvements.
- Responsive system.
- Trained manpower with expertise in handling cold chain products.
CHO = Chinese Hamster Ovary,
DCGI = Drug Controller General of India,
GMP = Good Manufacturing Practice,
ISO = International Organization for Standardization,
WHO = World Health Organization
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product"
References
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product"
References
1. Dr. Reddy's Laboratories Ltd (2016) Data on file. Unpublished
Key Features
Advantages Cresp®, the world's first biosimilar darbepoetin alfa, offers improved, convenient and affordable treatment for anemia associated with chronic kidney disease (CKD).
Product
Cresp® use improves quality of life and provides symptomatic relief of anemia (viz. from fatigue, reduced physical capacity, decreased cognition). Cresp® reduces the need for transfusions in patients with CKD and receiving hemodialysis, thus decreasing the risk of immunologic sensitization, infection and iron overload.
This means more advantages for both physicians and patients, such as:
- Convenience
A longer half-life means darbepoetin alfa can be administered less frequently than recombinant human erythropoietin (rHuEPO), with the same level of efficacy.[1-6] Darbepoetin alfa is administered only once a week, fortnightly or monthly as compared to three times a week with rHuEPO - Better patient compliance
Reducing the number of administrations required helps ensure better patient compliance, potentially improving treatment outcomes - Improved control
Darbepoetin alfa provides long-term control with minimal dose changes and a predictable rise in hemoglobin (Hb) level[2,4,5,7,8] - Flexible dosing and frequency
Dose flexibility allows weekly, fortnightly or monthly administration, with multiple strength availability, thus adequately meeting a patient's required dose titrations[2,4,5,7,8] - Ease of switching
Patients who have received rHuEPO can easily be switched over to the less frequent and more convenient darbepoetin alfa for maintenance[9-13]
Packaging
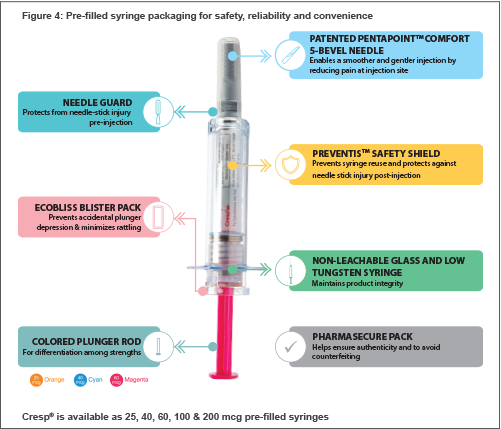
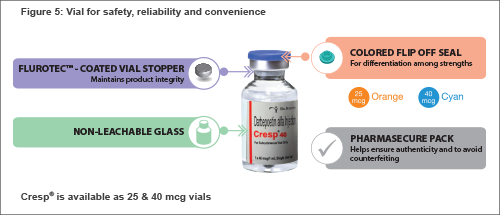
Both pre-filled syringes (PFS) and vials of Cresp® with several unique device features offer key advantages to physicians and patients.
Pre-Filled Syringe (PFS)
Vial Impact of Cresp®
Cresp® has been approved and commercially available in India since 2010. In more than five years since its launch, more than a million units of Cresp® have been manufactured, in compliance with current Good Manufacturing Practices (GMP), providing support to more than 30,000 CKD patients in India.[13]
The Reference Product, though approved in various countries across the world, is not available in most emerging markets, including India.[13]
Support Services
The "NephroConnect" App
The NephroConnect App is the first dedicated nephrology Android Application[13] and offers:
- Guidelines for managing anemia in CKD in simple tables and flow charts.
- Journal updates—a compilation of abstracts of most-read articles from world-renowned nephrology journals.
- Conferences updates and links.
- Cresp® information in detail.
- The Patient Corner—this includes information for patients about anemia in CKD, and important tips and lifestyle advice to help them manage it.
- Student Corner—a ready reckoner for avid students with useful calculators, important equations, drug dosages and case teasers.
The CHEER Program

When a patient is enrolled in CHEER, the program offers them the support they need, to help them make positive lifestyle changes related to nutrition and diet, and help them cope with the emotional stress that can often be overwhelming. The initiative touches on every aspect of patients' lives, and helps them understand and manage their condition better.
If you wish to enroll a patient in CHEER, please contact Dr. Reddy's scientific business officer.
App = Application,
CHEER = Committed to Help Educate and Encourage Renal patients,
CKD = chronic kidney disease,
EPO = erythropoietin,
GMP = Good Manufacturing Practice,
Hb = hemoglobin,
PFS = pre-filled syringes,
rHuEPO = recombinant human erythropoietin
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product"
References
We have referred to Aranesp® (Darbepoetin Alfa, Amgen, Thousand Oaks, California, USA) as the "Reference Product"
References
1. Aljama P et al. Nephrol Dial Transplant. 2001;16 Suppl 3:22-8.
2. Locatelli F et al. Kidney Int Suppl. 2008 Dec;(111):S33-7.
3. Locatelli F et al. Nephrol Dial Transplant. 2003 Feb;18(2):362-9.
4. Locatelli F et al. Kidney Int. 2001 Aug;60(2):741-7.
5. Vanrenterghem Y et al. Kidney Int. 2002 Dec;62(6):2167-75.
6. Mann J et al. Clin Nephrol. 2007 Mar;67(3):140-8.
7. Aranesp®, Amgen Package Insert
8. European Medicines Agency. Scientific discussion. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000332/WC500026142.pdf
9. Orazi E. G Ital Nefrol. 2008;25:223-226.
10. Summers S et al. Dial Transplant. 2005;34:358-362
11. Ardèvol M et al. Eur J Hosp Pharm Sci. 2006;12:47-51.
12. Biamino E et al. [abstract PUB284]. Presented at American Society of Nephrology Annual Congress, 27 October- 1 November 2004, St Louis, MO, USA
13. Dr. Reddy's Laboratories Ltd (2016) Data on file. Unpublished
14. Macdougall IC et al. Nephrol Dial Transplant. 2007 Jun;22 Suppl 4:iv2-iv9.
2. Locatelli F et al. Kidney Int Suppl. 2008 Dec;(111):S33-7.
3. Locatelli F et al. Nephrol Dial Transplant. 2003 Feb;18(2):362-9.
4. Locatelli F et al. Kidney Int. 2001 Aug;60(2):741-7.
5. Vanrenterghem Y et al. Kidney Int. 2002 Dec;62(6):2167-75.
6. Mann J et al. Clin Nephrol. 2007 Mar;67(3):140-8.
7. Aranesp®, Amgen Package Insert
8. European Medicines Agency. Scientific discussion. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000332/WC500026142.pdf
9. Orazi E. G Ital Nefrol. 2008;25:223-226.
10. Summers S et al. Dial Transplant. 2005;34:358-362
11. Ardèvol M et al. Eur J Hosp Pharm Sci. 2006;12:47-51.
12. Biamino E et al. [abstract PUB284]. Presented at American Society of Nephrology Annual Congress, 27 October- 1 November 2004, St Louis, MO, USA
13. Dr. Reddy's Laboratories Ltd (2016) Data on file. Unpublished
14. Macdougall IC et al. Nephrol Dial Transplant. 2007 Jun;22 Suppl 4:iv2-iv9.