About darbepoetin alfa
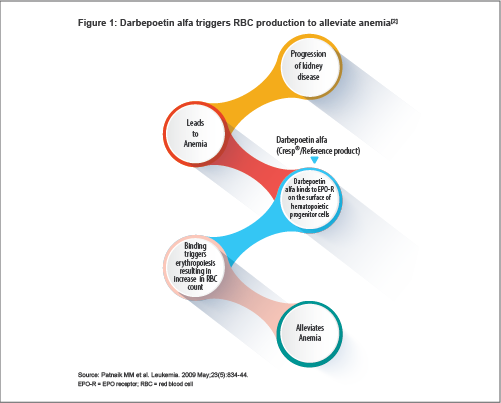
Darbepoetin alfa is a hyperglycosylated synthetic form of erythropoietin (EPO). It stimulates red blood cell (RBC) production by the same mechanism as the endogenous hormone: it binds to EPO receptors (EPO-Rs) on hematopoietic cells, resulting in the survival, differentiation, proliferation and maturation of RBCs[1] (see Figure 1). The resultant increase in RBCs alleviates anemia.
Darbepoetin alfa was first developed by Amgen (Thousand Oaks, California, USA) and approved by the US Food and Drug Administration and European Medicines Agency (EMA) for the treatment of anemia due to chronic kidney disease (CKD) in 2001[3].
It was the first major innovation in the treatment of anemia due to CKD after the discovery of recombinant human erythropoietin (rHuEPO).
Structure
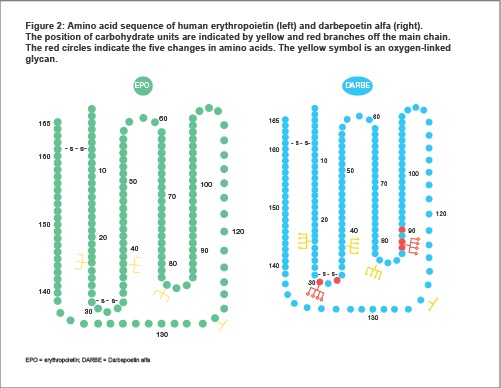
Darbepoetin alfa has a 165 amino acid backbone with two disulphide (-S-S-) bonds.
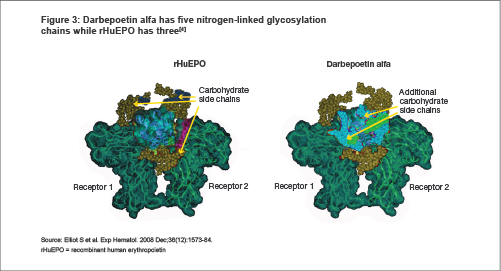
Darbepoetin alfa has two additional sites of glycosylation in comparison to rHuEPO. These sites are not directly involved in EPO-R binding.
This increased glycosylation resulted in darbepoetin alfa's predictably higher molecular mass, better biological activity profile, longer half-life, and delayed renal clearance[5,6] .
Mechanism of action
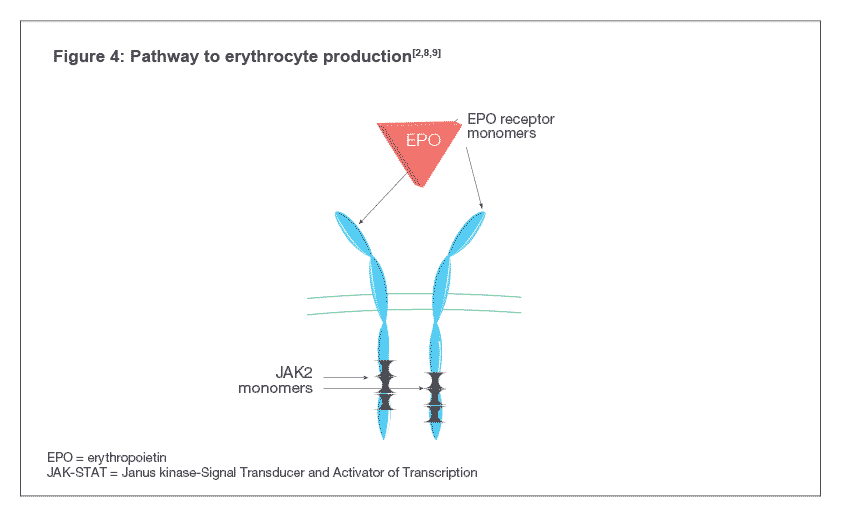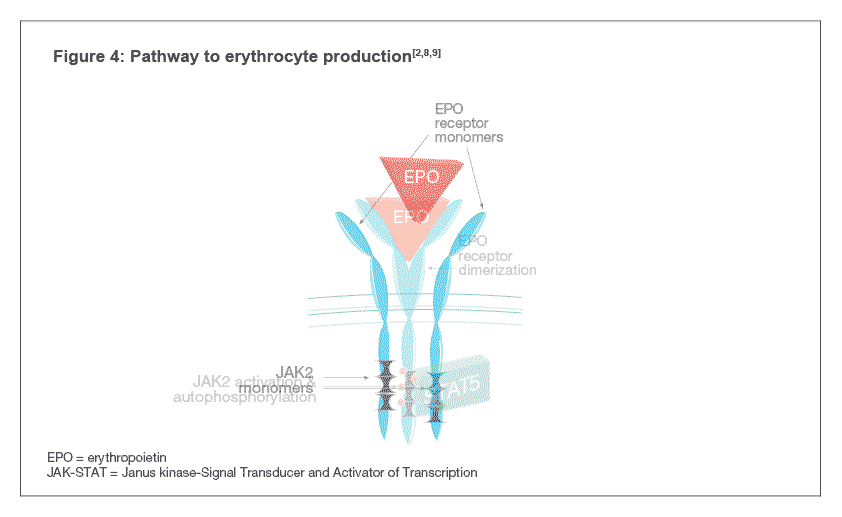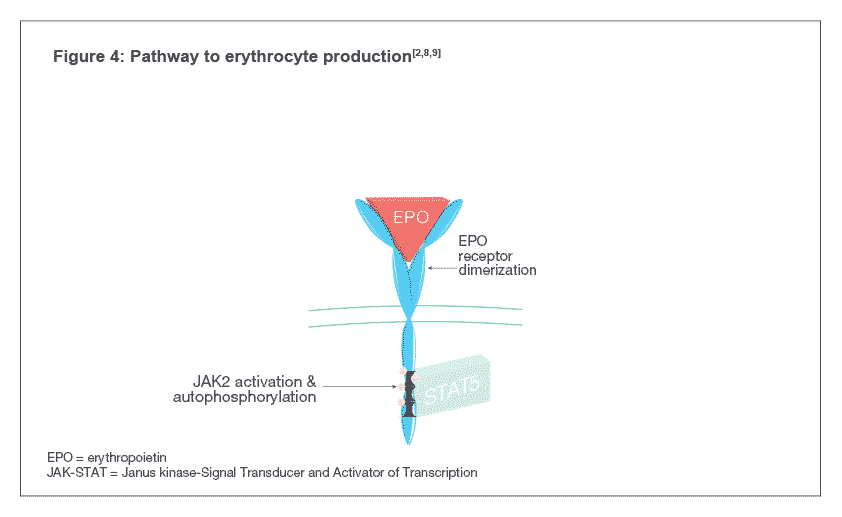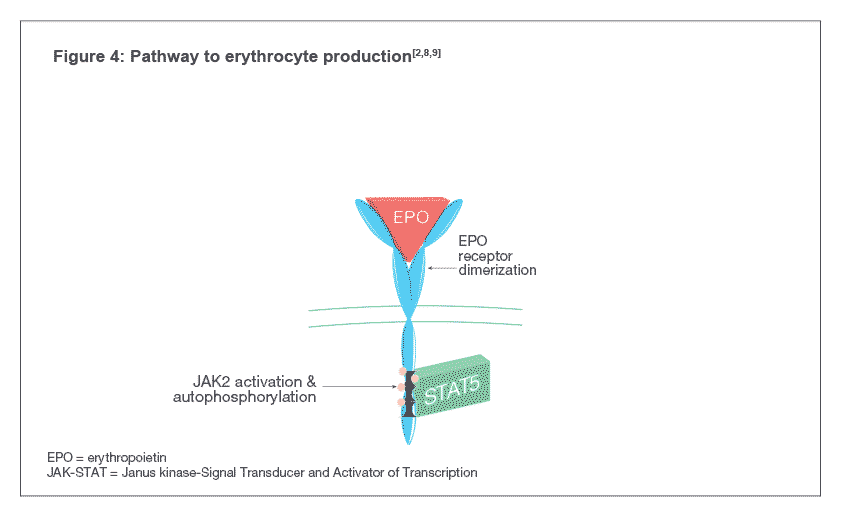
Darbepoetin alfa binds to EPO-Rs on the surface of hematopoietic progenitor cells and activates the Janus kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signal transduction pathway inside the cell, indicated by the phosphorylation of a protein called STAT5 (see Figure 4). The pathway causes the maturation of hematopoietic progenitor cells into RBCs[4,7] .
Darbepoetin alfa binds to EPO-Rs on the surface of hematopoietic progenitor cells which leads to receptor dimerization resulting in the activation of the Janus kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signal transduction pathway inside the cell, indicated by the phosphorylation of a protein called STAT5. The pathway causes the maturation of hematopoietic progenitor cells into RBCs [4,7].
Darbepoetin Alfa & rHuEPO
Half Life
Increased glycosylation is responsible for the increased half-life of darbepoetin alfa.
The concentration of circulating darbepoetin alfa thus exceeds the minimum required for stimulation of erythropoiesis for longer periods of time as compared with equivalent doses of rHuEPO
[10,11]. Subcutaneously administered darbepoetin alfa has a half-life two to three times longer than that of rHuEPO (about 48.8 hours)
[1,3,6,7,11] .
This allows physicians to dose darbepoetin less frequently than rHuEPO while maintaining an equivalent biological response for patients with CKD.
Darbepoetin alfa is comparable to rHuEPO
Darbepoetin alfa has the same onset of action and hemoglobin(Hb) response as rHuEPO.
Results from clinical studies in patients with chronic renal failure (CRF) either receiving or not receiving dialysis have shown that darbepoetin alfa is equivalent to rHuEPO in terms of onset of action, increases in Hb concentration, percentage of patients achieving target Hb concentration and average time to reach target Hb concentration, although darbepoetin alfa is administered less frequently (once weekly or every other week)
[12-14] .
Presentations
The active ingredient in Cresp
® is darbepoetin alfa (rDNA origin). Cresp
® is supplied in sterile preservative-free, non-pyrogenic single dose vials and pre-filled syringes (PFS).
- A single-dose vial of Cresp® contains either 25 or 40 mcg of the active substance darbepoetin alfa[10] .
- A single-dose PFS of Cresp® contains either 25, 40 or 60 mcg of the active substance darbepoetin alfa[10] .
For oncology indications, 100 mcg and 200 mcg PFS presentations are available.
Indications
- Treatment of symptomatic anemia associated with CRF in adults and pediatric patients, including patients on dialysis and patients not on dialysis[10].
- Also approved for treatment of symptomatic anemia in adult cancer patients with non-myeloid malignancies receiving chemotherapy[10] .
Dosing
For the treatment of symptomatic anemia in adult and pediatric CRF patients. Anemia symptoms and sequelae may vary with age, gender, and overall burden of disease; a physician's evaluation of the individual patient's clinical course and condition is necessary. Darbepoetin alfa should be administered subcutaneously in order to increase Hb to not greater than 12 g/dl (7.5 mmol/l). Due to intra-patient variability, occasional individual Hb values for a patient above and below the desired Hb level may be observed. Hb variability should be addressed through dose management, with consideration for the Hb target range of 10 g/dl (6.2 mmol/l) to12 g/dl (7.5 mmol/l). A sustained Hb level greater than 12 g/dl (7.5 mmol/l) should be avoided; guidance for appropriate dose adjustment for when Hb values exceeding 12 g/dl (7.5 mmol/l) are observed are described below. A rise in Hb level greater than 2 g/dl (1.25 mmol/l) over a four week period should be avoided. If it occurs, appropriate dose adjustment should be made as provided. Treatment with darbepoetin alfa is divided into two stages—correction and maintenance phase. Guidance is given separately for adult and pediatric patients. Treatment of pediatric patients younger than 1 year of age has not been studied.
Adult patients with chronic renal failure[10]
Correction Phase
The initial dose by subcutaneous administration is 0.45 mcg/kg body weight, as a single injection once weekly. Alternatively, inpatients not on dialysis, an initial dose of 0.75 mcg/kg may be administered subcutaneously as a single injection once every two weeks. If the increase in Hb is inadequate (less than 1 g/dl (0.6 mmol/l) in four weeks) increase the dose by approximately 25%. Dose increases must not be made more frequently than once every four weeks. If the rise in Hb is greater than 2 g/dl (1.25 mmol/l) in four weeks reduce the dose by approximately 25%. If the Hb exceeds 12 g/dl (7.5mmol/l), a dose reduction should be considered. If the Hb continues to increase, the dose should be reduced by approximately 25%. If after a dose reduction, Hb continues to increase, the dose should be temporarily withheld until the Hb begins to decrease, at which point therapy should be reinitiated at approximately 25% lower than the previous dose. The Hb should be measured every one or two weeks until it is stable. Thereafter the Hb can be measured at longer intervals.
Maintenance Phase
In the maintenance phase, darbepoetin alfa may continue to be administered as a single injection once weekly or once every two weeks. Dialysis patients converting from once weekly to once every other week dosing with darbepoetin alfa should initially receive a dose equivalent to twice the previous once weekly dose. In patients not on dialysis, once the target Hb has been achieved with once every two week dosing, darbepoetin alfa may be administered subcutaneously once monthly using an initial dose equal to twice the previous once every two week dose. Dosing should be titrated as necessary to maintain the Hb target. If a dose adjustment is required to maintain Hb at the desired level, it is recommended that the dose is adjusted by approximately 25%. If the rise in Hb is greater than 2 g/dl (1.25 mmol/l) in four weeks reduce the dose by approximately 25%, depending on the rate of increase. If the Hb exceeds 12 g/dl (7.5 mmol/l), a dose reduction should be considered. If the Hb continues to increase, the dose should be reduced by approximately 25%.
If after a dose reduction, Hb continues to increase, the dose should be temporarily withheld until the Hb begins to decrease, at which point therapy should be reinitiated at approximately 25% lower than the previous dose. Patients should be monitored closely to ensure that the lowest approved dose of darbepoetin alfa is used to provide adequate control of the symptoms of anemia. After any dose or schedule adjustment the Hb should be monitored every one or two weeks. Dose changes in the maintenance phase of treatment should not be made more frequently than every two weeks.
Clinical studies have demonstrated that adult patients receiving rHuEPO one, two or three times weekly may be converted to once weekly or once every other week darbepoetin alfa. The initial weekly dose of darbepoetin alfa (mcg/week) can be determined by dividing the total weekly dose of rHuEPO (IU/week) by 200. The initial every other week dose of darbepoetin alfa (mcg/every other week) can be determined by dividing the total cumulative dose of rHuEPO administered over a two-week period by 200. Because of individual variability, titration to optimal therapeutic doses is expected for individual patients. When substituting darbepoetin alfa for rHuEPO the Hb should be monitored every one or two weeks and the same route of administration should be used.
Pediatric patients with chronic renal failure[10]
Correction Phase
For patients ≥ 11 years of age, the initial dose by subcutaneous administration is 0.45 mcg/kg body weight, as a single injection once weekly. Alternatively, in patients not on dialysis, an initial dose of 0.75 mcg/kg may be administered subcutaneously as a single injection once every two weeks. If the increase in Hb is inadequate (less than 1 g/dl (0.6 mmol/l) in four weeks) increase the dose by approximately 25%. Dose increases must not be made more frequently than once every four weeks. If the rise in Hb is greater than 2 g/dl (1.25 mmol/l) in four weeks reduce the dose by approximately 25%, depending on the rate of increase. If the Hb exceeds 12 g/dl (7.5 mmol/l), a dose reduction should be considered. If the Hb continues to increase, the dose should be reduced by approximately 25%. If after a dose reduction, Hb continues to increase, the dose should be temporarily withheld until the Hb begins to decrease, at which point therapy should be reinitiated at approximately 25% lower than the previous dose. The Hb should be measured every one or two weeks until it is stable. Thereafter the Hb can be measured at longer intervals. No guidance regarding the correction of Hb is available for pediatric patients 1 to 10 years of age.
Maintenance Phase
For pediatric patients ≥ 11 years of age, in the maintenance phase, darbepoetin alfa may continue to be administered as a single injection once weekly or once every two weeks. Dialysis patients converting from once weekly to once every other week dosing with darbepoetin alfa should initially receive a dose equivalent to twice the previous once weekly dose. In patients not on dialysis, once the target Hb has been achieved with once every two week dosing, darbepoetin alfa may be administered subcutaneously once monthly using an initial dose equal to twice the previous once every two week dose. For pediatric patients 1-18 years of age, clinical data in pediatric patients has demonstrated that patients receiving rHuEPO two or three times weekly may be converted to once weekly darbepoetin alfa, and those receiving rHuEPO once weekly may be converted to once every other week darbepoetin alfa. The initial weekly pediatric dose of darbepoetin alfa (mcg/week) can be determined by dividing the total weekly dose of rHuEPO (IU/week) by 240. The initial every other week dose of Darbepoetin alfa (mcg/every other week) can be determined by dividing the total cumulative dose of rHuEPO administered over a two-week period by 240. Because of individual variability, titration to optimal therapeutic doses is expected for individual patients. When substituting darbepoetin alfa for rHuEPO, Hb should be monitored every one or two weeks and the same route of administration should be used. Dosing should be titrated as necessary to maintain the Hb target. If a dose adjustment is required to maintain Hb at the desired level, it is recommended that the dose is adjusted by approximately 25%. If the rise in Hb is greater than 2 g/dl (1.25 mmol/l) in four weeks, reduce the dose by approximately 25% depending on the rate of increase. If the Hb exceeds 12 g/dl (7.5 mmol/l), a dose reduction should be considered. If the Hb continues to increase, the dose should be reduced by approximately 25%. If after a dose reduction, Hb continues to increase, the dose should be temporarily withheld until the Hb begins to decrease, at which point therapy should be reinitiated at approximately 25% lower than the previous dose. Patients should be monitored closely to ensure that the lowest approved dose of darbepoetin alfa is used to provide adequate control of the symptoms of anemia. After any dose or schedule adjustment, the Hb should be monitored every one or two weeks. Dose changes in the maintenance phase of treatment should not be made more frequently than every two weeks.
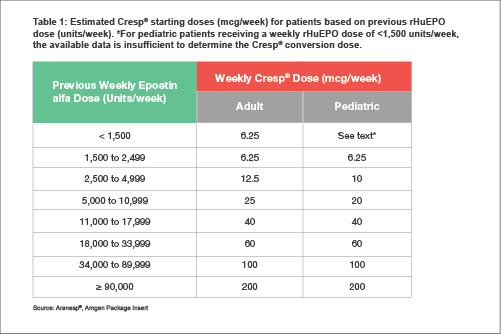
Dose calculation for switching patients receiving rHuEPO to darbepoetin alfa can be achieved following the guidance
[15] given in
Table 1.
CKD = chronic kidney disease, CRF = chronic renal failure, EMA = European Medicines Agency, EPO = erythropoietin, EPO-R = erythropoietin receptor, Hb = hemoglobin, IU = International Units, JAK-STAT = Janus kinase-Signal Transducer and Activator of Transcription, PFS = pre-filled syringe, RBC = red blood cell, rDNA = recombinant deoxyribonucleic acid, rHuEPO = recombinant human erythropoietin
References
1. Macdougall IC et al. J Am Soc Nephrol. 1999 Nov;10(11):2392-5.
2. Patnaik MM et al. Leukemia. 2009 May;23(5):834-44.
3. Powell J et al. Proc (Bayl Univ Med Cent). 2002 Jul;15(3):332-5.
4. Elliot S et al. Exp Hematol. 2008 Dec;36(12):1573-84.
5. Macdougall IC et al. Nephrol Dial Transplant. 2007 Jun;22 Suppl 4:iv2-iv9.
6. Agarwal AK. Expert Opin Drug Saf. 2009 Mar;8(2):145-53.
7. Egrie JC et al. Exp Hematol. 2003 Apr;31(4):290-9.
8. Moore E, Bellomo R. Ann Intensive Care. 2011 Mar 21;1(1):3.
9. Zhang Y et al. Int J Mol Sci. 2014 Jun 10;15(6):10296-333.
10. Cresp®, Dr. Reddy's Package Insert
11. Macdougall IC. Nephrol Dial Transplant. 2002;17 Suppl 5:66-70.
12. Locatelli F et al. Kidney Int Suppl. 2008 Dec;(111):S33-7.
13. Locatelli F et al. Kidney Int. 2001 Aug;60(2):741-7.
14. Vanrenterghem Y et al. Kidney Int. 2002 Dec;62(6):2167-75.
15. Aranesp®, Amgen Package Insert.